Abstract
AML is a heterogenous disease in which prognosis and treatment is determined by recurrent genetic mutations and chromosomal abnormalities. Some genetic alterations result in aberrant RNA translation, which is exploited by leukemia stem cells (LSCs) to produce short-lived oncoproteins (c-Myc, Mcl-1) to promote cell survival. Since LSCs are considered as the source of treatment failure and relapse, it is critical to understand how LSCs hijack the translational machinery in order to develop effective therapeutics in AML.
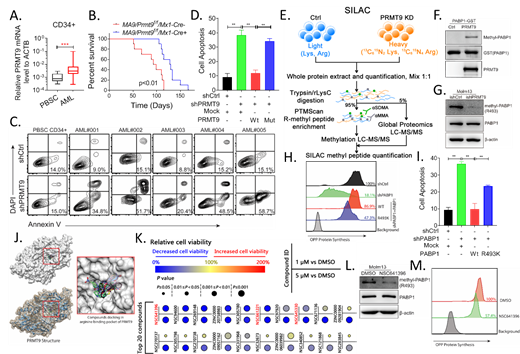
Arginine methylation catalyzed by protein arginine methyltransferases 1-9 (PRMT1-9) regulates various activities, including RNA translation. We revealed that PRMT1 over activation contributes to leukemia maintenance (Blood, 2019; Blood, 2019). Unlike PRMT1, the recently described PRMT9, is not defined in leukemia. Follow up of AML patients from existing datasets (GSE12417, TARGET) confirms that patients with higher levels of PRMT9 correlates with decreased overall survival. In our AML cohort, we observed significantly increased PRMT9 mRNA levels in AML CD34 + cells relative to normal counterparts (Fig. 1A), spurring our interest in evaluating its function in AML. We thus developed a Prmt9 conditional KO mouse (Mx1-Cre/Prmt9 f/f) and crossed it with an MLL-AF9 (MA9) knock-in mouse to generate Prmt9-KO/MA9 mice for further transplant assay. As a result, Prmt9-KO significantly delayed leukemogenesis in recipient mice carrying MA9 transplants evidenced by prolonged survival (Fig. 1B). Prmt9-KO impaired leukemia-initiating activity evidenced by secondary transplantation that significant less residual CD45.2 + MA9 leukemia cells were found in BM of secondary recipients receiving Prmt9-KO cells relative to those of Prmt9-WT controls. We next validated PRMT9 function in human AML. PRMT9-KD potentially impaired survival of primary AML CD34 + cells, whereas large sparing normal counterparts (Fig. 1C). Moreover, we designed a WT PRMT9 construct and corresponding catalytically dead mutant, both resistant to shPRMT9 (WT-R and MUT-R). Unlike MUT-R, WT-R expression rescued survival effects seen following shPRMT9 treatment, indicating that PRMT9 catalytic activity is required for AML survival (Fig. 1D). To define PRMT9 downstream effectors, we searched for methylated substrates in Molm13 with inducible PRMT9-KD or corresponding controls by performing SILAC analysis coupled with quantitative Mass-Spec (MS) (Fig. 1E). Complete analyses of normalized methyl peptides SILAC ratios revealed more prominent down-regulation of R-methylation in PRMT9-KD cells, with 49 methyl sites downregulated. Notably, methylated PAPB1 C-terminus peptide containing R493 was most significantly depleted upon PRMT9-KD. PABP1 potentiates translation initiation by binding to the poly(A) mRNA tail and interacting with factors like eIF4G. We thus generated an anti-R493 methylation antibody. Indeed, PRMT9 was confirmed to catalyze R493 methylation through in-vitro and cellular methylation assays (Fig. 1F, G). To define the function of R493 methylation, we ectopically expressed either WT PABP1 or the R493K mutant in Molm13 cells and then engineered those cells to express shPABP1 to KD endogenous PABP1. Notably, unlike WT PABP1, expression of R493K suppressed protein synthesis and increased apoptosis after endogenous PABP1-KD, suggesting that PRMT9 mediated PABP1-R493 methylation promotes AML viability (Fig. 1H, I).
To discover PRMT9 inhibitors, we conducted a structure-based virtual screen of 960,000 compounds from NCI and Zinc compound libraries (Fig. 1J). We requested the top 300 candidates to assess their anti-leukemia activity in Molm13 cells (Fig. 1K). The top 20 most effective compounds were further analyzed via PRMT9 methylation assay (R493 antibody detection). NSC641396 exhibited the most potent PRMT9 inhibitory effects, as evidenced by decreased PABP1 R493 methylation levels at a dose <1 µΜ (Fig. 1L). NSC641396 treatment on AML CD34 + cells altered polysome profiling, decreased protein biosynthesis and reduced levels of short-lived proteins, suggesting translation inhibition (Fig. 1M).
Taken together, our results suggest that PRMT9 plays an oncogenic role in AML or LSC maintenance, by promoting PABP1 methylation mediated translation activity. Further studies are needed to explore if targeting PRMT9 with newly identified lead compound ablates LSCs activity.
Marcucci: Novartis: Other: Speaker and advisory scientific board meetings; Agios: Other: Speaker and advisory scientific board meetings; Abbvie: Other: Speaker and advisory scientific board meetings.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal